How to Make Design Controls More Efficient With Traceability
Matrix
by Alec Alpert
⇓ Download this article as PDF
If you are a manufacturer of Class III, Class II, and certain Class I medical devices, you must meet mandatory Food and Drug Administration requirements for design controls, namely 21 CFR 820.30. Companies that have ISO 13485 and/or ISO 9001 certifications must comply with design control requirements as well.
Design controls are practices, policies, and procedures that formally govern the design and development process for a medical device. They provide core teams with step-by-step directions for developing the product from the user needs assessment to product commercialization. They create a concrete list of project deliverables, due dates, and topics for discussions at formal design reviews.
Let’s say you are developing a sophisticated medical device with a long list of customer requirements, which in the world of regulations are called “design input.” Once you have translated those customer requirements into the product requirements and product specifications, you have established the scope of your “design output.”
Your complex product may consist of thousands of components and multiple subassemblies, for example, an MRI machine, or a linear accelerator, or an anesthesia system. Because of the product complexity, most likely, it would also require standalone specifications for subassemblies and system-level specifications as well, including those for hardware, software, and perhaps even firmware.
As it typically takes years to complete new product development, you need to keep track of the customer requirements (design input) through implementation of the product requirements/specifications (design output). In other words, at the end of the product development cycle, design output must meet the design input; the product must demonstrate via design verification and validation, clinical trials, etc., that all customer requirements have been met, and it is safe and effective.
Then a natural question arises:
How to keep track of customer requirements, hundreds of specifications, and their verification and validation status?
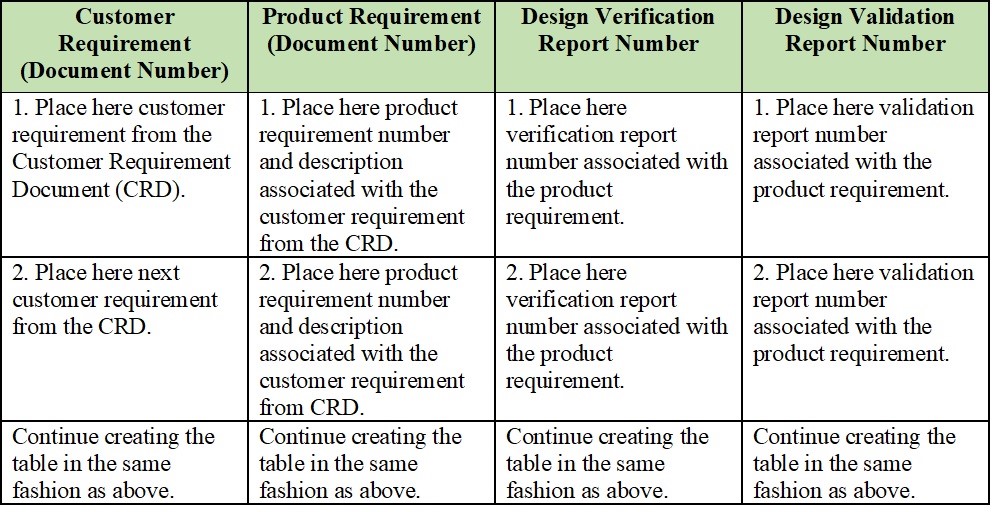
One solution could be having a formal Traceability Matrix document linking customer requirements (design input) with the product requirements and their respective design verification and validation documentation (design output). It would be constantly updated to reflect the current state of affairs.
The advantages of having Traceability Matrix document are that you:
• Maintain at a glance visibility of design input and design output status.
• Maintain good communication and collaboration within the core team.
• Improve compliance with the design controls requirements.
• Improve the odds of successful audits.
• Have an overall design management tool.
Even if your product is not as complicated as an MRI machine, the Traceability Matrix is still a valuable design management tool.
In its basic form, the Traceability Matrix would look like this:
Perhaps you already use some form of the Traceability Matrix, but if you don’t, you can easily develop a procedure with its associated Traceability Matrix template. This procedure would include such key sections as:
• Purpose
• Scope
• Applicable documents
• Responsibilities
• Definitions
• Product description
• Procedure how to create and maintain Traceability Matrix
The Traceability Matrix document would be a controlled document filed in the design history file (DHF) among other project deliverables.