HELPING YOU ESTABLISH AND MAITAIN RISK MANAGEMENT
FILE
 I can help you establish and maintain a Risk Management File in compliance with your procedures and policies, industry standards (ISO 14971, ISO 13485, IEC 60601) and regulations (21CFR 820).
I can help you establish and maintain a Risk Management File in compliance with your procedures and policies, industry standards (ISO 14971, ISO 13485, IEC 60601) and regulations (21CFR 820).
Here are the highlights of what I can do for your Risk Management File:
1. Prepare and execute a Risk Management Plan that will describe the methodologies employed in risk management planning, analysis, evaluation, control, and reporting.
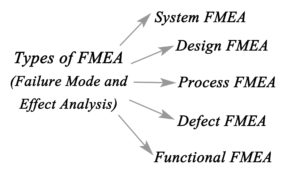
2. Facilitate the Hazard Analysis process, including Design FMEA, Use FMEA and Process FMEA.
3. Prepare and maintain Hazard Analysis Reports that will contain typical items, such as:
• Failure modes.
• Failure effects.
• Failure causes.
• Severity.
• Probability of occurrence.
• RPN number.
• Risk mitigation.
• Evaluation of residual risk—risk/benefit assessment.
4. Prepare a Risk Management Report that will document the risk management process, including a confirmation that all risks have been minimized as much as possible. Additionally, the Risk Management Report will establish and document a plan to collect, review, and evaluate information about the product or similar products in production and post-production phases.
5. Create and maintain the Risk Managemant file in the Design History File (DHF).
To discuss how I can help your with Risk Management, contact me here.